Gene editing promises cures for a wide range of diseases. Zinc-finger nucleases (ZFNs) were developed as the first programmable editors in the early 2000s, followed by TALENs (Transcription Activator Like Effector Nucleases) about a decade later[1]. However, it was the discovery and application of easy to use CRISPR/Cas9 gene editing methods, that sparked a flurry of new activity since 2013. New gene editing methods that build on this platform continue to be a hot area of research, with the goal of developing techniques that increase specificity and circumvent the need for double-stranded breaks (DBSs) in order to reduce off-target effects and minimize safety concerns[2]. Moreover, increasing the size of sequences that can be modified, and improving editing efficiency in non-dividing cells are also areas of great interest.
PXB-cells are high quality primary human hepatocytes (PHHs) isolated from the liver of a chimeric mouse with a humanized liver, the PXB-mouse[3]. They are stable in culture for 28 days or more and have the distinct advantage of continuous production from a single donor for periods of several years. This minimizes the time needed by the user to validate new lots of PHHs and enhances reproducibility. Recently, a number of studies have been published that showcase the advantages of PXB-cells an in vitro platform for studying novel gene editing tools.
Targeting HBV with Base Editing
Base editors have the ability to catalyze deamination and convert one nucleotide into another without requiring the introduction of a double-strand break in the genome. Thus, multiple edits can be introduced in a single cell with minimal genomic rearrangements, resulting in a lower chance of unintended mutations, insertions, or deletions. Beam Therapeutics recently published a paper testing whether a base editing approach could be used to develop more effective therapeutics for Hepatitis B virus (HBV)[4]. Chronic infection with HBV can result in cirrhosis of the liver, eventually leading to liver failure. While an effective vaccine exists an estimated 254 million people are currently infected with HBV around the globe, and about 1.2 million more become infected each year[5]. One of the challenges in curing HBV is due to the virus’ integration in the genome and the production of covalently closed circular DNA (cccDNA), which acts as a reservoir of viral genome in the hepatocyte and has proven intractable to currently available therapeutics. Smekalova et al. developed a Cytosine Base Editor (CBE) known as BE4 to target cccDNA. This editor converts cytosine (C) to thymine (T) and lead to gene silencing by introducing stop codons. Two guide RNAs were incorporated in the CBE to target both cccDNA and integrated HBV DNA for maximal efficacy. Experiments in HBV-infected PXB-cells showed that transfecting the CBE at 5- and 12-days post-infection led to a sustained reduction in levels of HBsAg, HBeAg, extracellular HBV DNA, total HBV DNA and 3.5-kb RNA (Figure 1 A-C). Moreover, cccDNA levels were not affected showing that the CBE works by silencing gene expression through the introduction of mutations, rather than changing stability of the cccDNA itself (Figure 1 D). Finally, levels of C to T editing were measured using next generation sequencing, confirming the editor was working as anticipated (Figure 1 E).
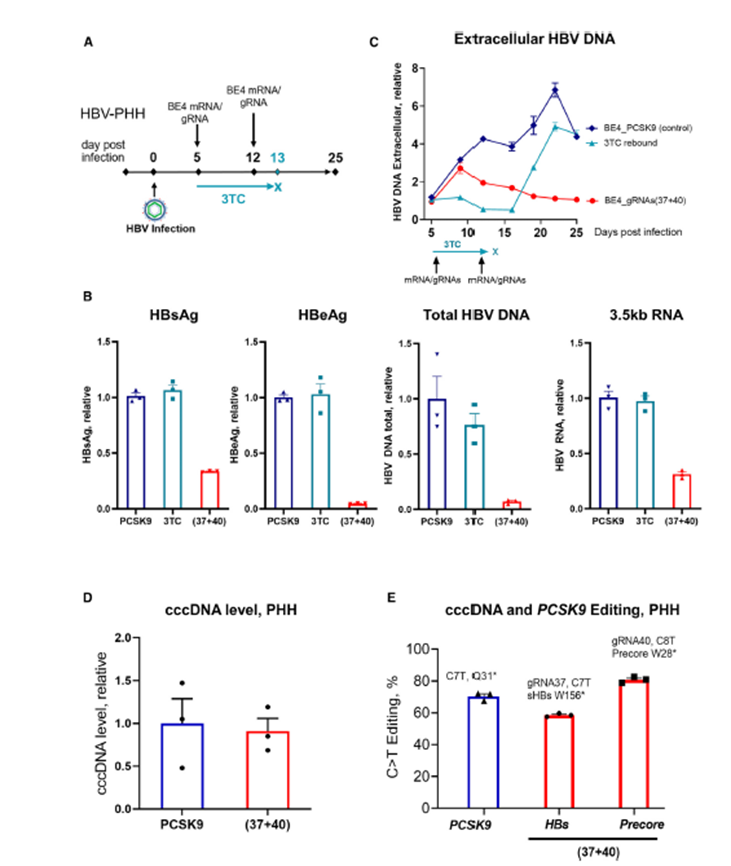
Figure 1: Antiviral efficacy of base editing in HBV- infected PHHs (PXB-cells) (A) Experimental scheme. (B) Multiplexing the 2 gRNAs with BE4 simultaneously reduces HBsAg, HBeAg, 3.5-kb RNA, and total HBV DNA. Viral parameters assessed at the end of the experiment, day 25 post infection. BE4 with the PCSK9 gRNA was used as a control for normalizing the data. (C) HBV replication assessed by HBV DNA qPCR in PHH supernatant. Discontinuation of 3TC (Lamivudine) leads to HBV rebound, whereas base editing prevents viral rebound. (D) cccDNA level was assessed by qPCR on the DNA samples pretreated with ExoI/III. (E) Level of the C>T functional editing that leads to the introduction of the stop codons in HBs and Precore genes, assessed by NGS on ExoI/III treated cccDNA samples as well as PCSK9 (assessed on total DNA). Data are represented as mean ± SEM for n = 3. Adapted from Smekalova et al., 2023
Overall, PXB-cells provided key data to show that a CBE approach can be useful to target cccDNA during HBV infection, and this finding was corroborated by data in other cellular as well as in vivo models.
Novel Methods to Tackle Large Scale Gene Editing
While base editors are great tools for making specific single nucleotide changes, insertion of large DNA fragments into the genome has long been a goal in the development of gene therapy. Two recent papers from the AbuGoot Lab at MIT detail novel approaches to tackle this problem. The first, Programmable Addition via Site-specific Targeting Elements (PASTE) is a genome editing platform that enables precise integrations of large DNA sequences (up to 36kb) into specific genomic loci without DSBs6. In order to develop PASTE, Yarnall et al combined features of CRISPR/Cas9 editing with serine integrases to develop a tool that can easily be reprogrammed for any genomic target. Importantly, experiments in PXB-cells showed that PASTE is active in non-dividing, primary cells when delivered with viral vectors (AAV and Adenovirus) (Figure 2 A). Further experiments demonstrated successful in vivo gene editing.
A second approach, STICHR, harnesses the features of retrotransposons, specifically non-long terminal repeats (nLTRs), which are naturally occurring transposable DNA elements that have evolved to jump from place to place in the genome via an RNA intermediate. Like PASTE, this system is programmable, but has the added benefit that it also enables scarless genome editing and allows a broad range of editing types including single base edits, larger gene deletions (up to 12.7 kb) and replacements and multiplexed insertion from RNA templates[7]. The method was demonstrated to work with moderate efficiency in both human cell lines and non-dividing primary human hepatocytes (PXB-cells) (Figure 2B).
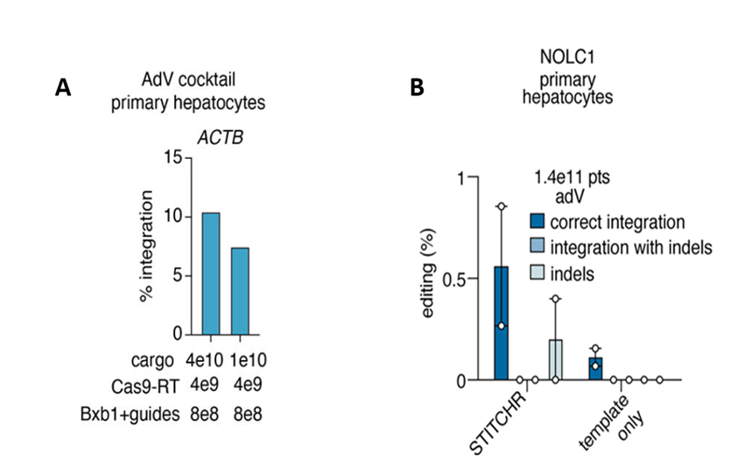
Figure 2: (A) PASTE editing in PXB-cells - Integration efficiency of AdV delivery of integrase (Bxb1), guides, and cargo in primary human hepatocytes (PXB-cells). Viral components were listed at dosages indicated. (n= 1). (B) STITCHR editing - EGFP insertion by SpCas9H840A-R2ToccΔ1-169 at NOLC1 in quiescent primary human hepatocytes (PXB-cells) compared to SpCas9H840A control. 1.4e11 viral copies was used in the dual vector condition; half of that for the single vector payload only condition. Editing quantified by NGS. Adapted from Yarnall et al., 2023 and Fell et al., 2025.
These new gene editing methods show promise for therapeutic development in a wide variety of diseases as they can be used to replace entire genes, or introduce regulatory elements to modulate expression of genes. Both these methods were shown to work effectively in PXB-cells, an important step in showing applicability to human, non-dividing cells, which would make up many of the target tissues for therapeutics.
Reversible Gene Silencing with Epigenetic Editing (Tremblay et al., 2025)
Epigenetic editing has recently emerged as a method that can have durable therapeutic effects without changing the underlying DNA sequence. Instead, this approach works by silencing disease-causing genes through the introduction of DNA methylation at CpG dinucleotide sites in promoter regions of the target, without the need to introduce a cut or nick in the DNA[2,8]. Moreover, unlike traditional gene editing approaches, epigenetic editing is also theoretically reversable by using activators designed to remove methyl marks on CpGs. Tuning of target gene expression to optimal levels could even be possible, by using combinations of silencers and activators in the same cell or tissue.
nChroma Therapeutics recently developed and characterized a novel PCSK9 epigenetic editor (EE)[8]. PCSK9 is part of a pathway that modulates the levels of the LDL (low density lipoprotein) receptor on the surface of hepatocytes. Increased PCSK9 activity reduces the levels of the LDLR and leads to higher levels of circulating LDL cholesterol – a cause of atherosclerosis which leads to increased risk of stroke and heart attack[9]. Mutations in the PCSK9 gene can lead to familial hypercholesterolemia, a genetic disease where patients have elevated levels of LDL cholesterol, and a significantly increased risk of coronary artery disease. Thus, therapeutic approaches that lower levels of PCSK9 could be helpful for a wide range of patients.
In this study, the authors carried out guide RNAs screening in HeLa cells to identify which would be the most effective to target human PCSK9. PXB-cells were then used to validate the results of the screen and further show efficacy and specificity of the editing. The guide RNAs and EE mRNA were formulated into lipid nanoparticles (LNPs) for delivery. In combination with the EE, the top five guide RNAs identified in the screen all showed a durable decrease in PCSK9 secretion that lasted up to two weeks in culture (Figure 3). This was in contrast to using a CRISPRi approach, which showed rebound after Day 7.
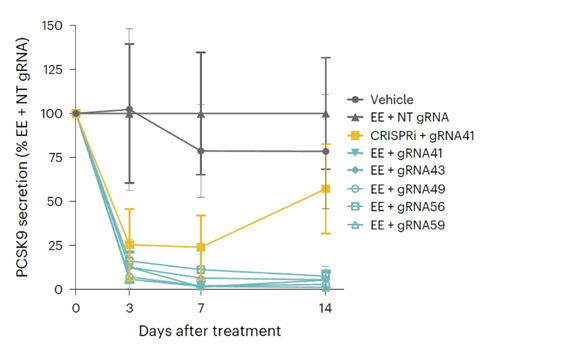
Figure 3: Comparison of efficacy of top 5 gRNAs/EE in PXB-cells. Delivery was via LNP and vehicle or NT gRNA were used as negative controls. CRISPRi approach is also included. Durable decrease of PCSK9 protein secretion was achieved with all the guides in combination with the EE, while CRISPRi showed rebound after Day 7. Adapted from Tremblay et al., 2025.
To further confirm the specificity of the silencing, deep-read RNA sequencing was used to look for off-target changes in methylation in PXB-cells treated with the PCSK9-EE. No significant changes in gene expression or global methylation were identified in the primary human hepatocytes (Figure 4 A, B). Moreover, while some increases in CpG methylation were seen at sites other than the PCSK9 locus, none of these correlated with meaningful changes in gene expression (Figure 4 C,D). Whole-genome methylation sequencing (WGMS) was also carried out and confirmed that the activity of the EE was specific to PCSK9 (Figure 4 E, F). Further experiments showed this PCSK9-EE could achieve reversible silencing of hPCSK9 in transgenic mice with durability of at least one year. Potent silencing was also observed in NHPs. While these in vivo studies were important to show activity, potency and durability, PXB-cells provided an essential platform to both identify a highly active PCSK9-EE and to confirm the specificity of the epigenetic editing.
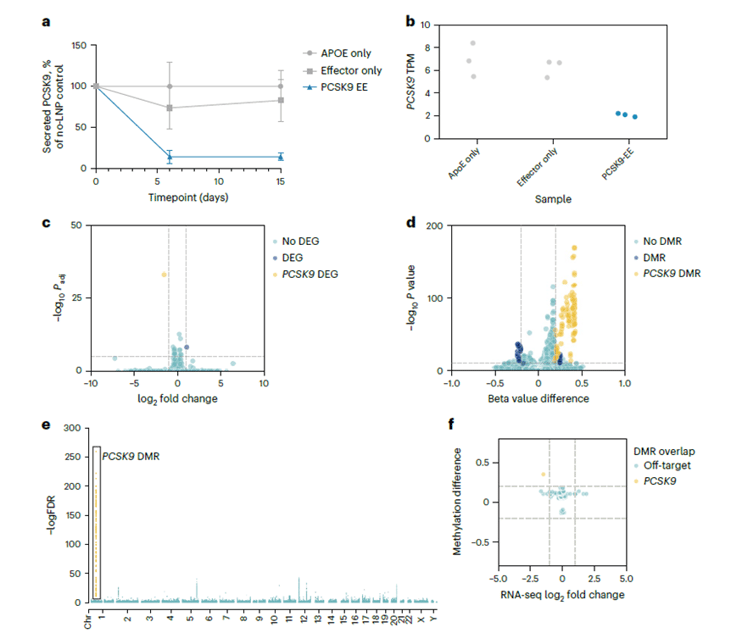
Figure 4: (A) The LNP formulation of PCSK9-EE specifically reduced levels of secreted PCSK9 protein levels in PXB-cells at 6 d and 15 d after treatment. APOE or effector only are negative controls. (B) Specificity testing was assessed using RNA-seq on three independent replicates of each control condition (ApoE Only and Effector Only) and PCSK9-EE. On-target PCSK9 TPM from RNA-seq for each replicate are shown in the dot plot. (C) Volcano plot of RNA-seq data comparing PCSK9-EE versus Effector Only control. (D) Specificity of methylation at CpG-enriched sites was measured using a Twist Human Methylome Hybrid Capture Methylation Sequencing assay. Volcano plot of CpG methylation comparing PCSK9-EE versus Effector Only control. (E) Manhattan plot of genomewide methylation, as determined by a WGMS assay, comparing PCSK9-EE versus Effector Only control. (F) Scatterplot showing methylation difference of DMRs from WGMS (y axis) versus log2FC from RNA-seq (x axis) of all genes within 20 kb of each DMR for the PCSK9-EE versus Effector Only control comparison. DEG, differentially expressed gene.
Conclusion
Novel gene editing platforms are important to develop new therapeutics and identify innovative ways of targeting intractable diseases. Validation of these new approaches requires a high-quality in vitro model that can give reliable data about efficacy, durability, and specificity of the editing platform. PXB-cells are emerging as a key tool for in vitro validation of emerging gene editing approaches, with a proven track record of providing strong, translatable human data with applications for initial development of editors, disease modeling and specificity testing.
References
- Carroll D. A short, idiosyncratic history of genome editing. Gene and Genome Editing. 2021:100002. doi.org/10.1016/j.ggedit.2021.100002
- Liu D, Cao D, Han R. Recent advances in therapeutic gene-editing technologies. Mol Ther. 2025;33(6):2619-2644. doi:10.1016/j.ymthe.2025.03.026
- Yamasaki C, Ishida Y, Yanagi A, et al. Culture density contributes to hepatic functions of fresh human hepatocytes isolated from chimeric mice with humanized livers: Novel, long-term, functional two-dimensional in vitro tool for developing new drugs. PLoS One. 2020;15(9):e0237809. Published 2020 Sep 11. doi:10.1371/journal.pone.0237809
- Smekalova EM, er. al. Cytosine base editing inhibits hepatitis B virus replication and reduces HBsAg expression in vitro and in vivo. Mol Ther Nucleic Acids. 2023 Dec 27;35(1):102112. doi: 10.1016/j.omtn.2023.102112
- https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- Yarnall MTN et al. Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases. Nat Biotechnol. 2023 Apr;41(4):500-512. doi: 10.1038/s41587-022-01527-4
- Fell CW et al. Reprogramming site-specific retrotransposon activity to new DNA sites. Nature. 2025 Jun;642(8069):1080-1089. doi: 10.1038/s41586-025-08877-4
- Tremblay F, Xiong Q, Shah SS, et al. A potent epigenetic editor targeting human PCSK9 for durable reduction of low-density lipoprotein cholesterol levels. Nat Med. 2025;31(4):1329-1338. doi:10.1038/s41591-025-03508-x
- Peterson AS, Fong LG, Young SG. PCSK9 function and physiology. J Lipid Res. 2008;49(7):1595-1599. doi:10.1194/jlr.cx00001-jlr200
Figures
