Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in developed countries, with an estimated incidence of around 25% worldwide.
This liver disease often occurs in people with obesity and metabolic syndrome. About 20% of individuals with NAFLD will progress to non-alcoholic steatohepatitis or NASH, the most severe form of the disease, which is diagnosed once the liver has advanced from simple fat deposits to serious inflammation, cell damage, and fibrosis. NASH can eventually lead to cirrhosis and liver cancer, and is a leading cause of liver transplant in the United States[1][2].
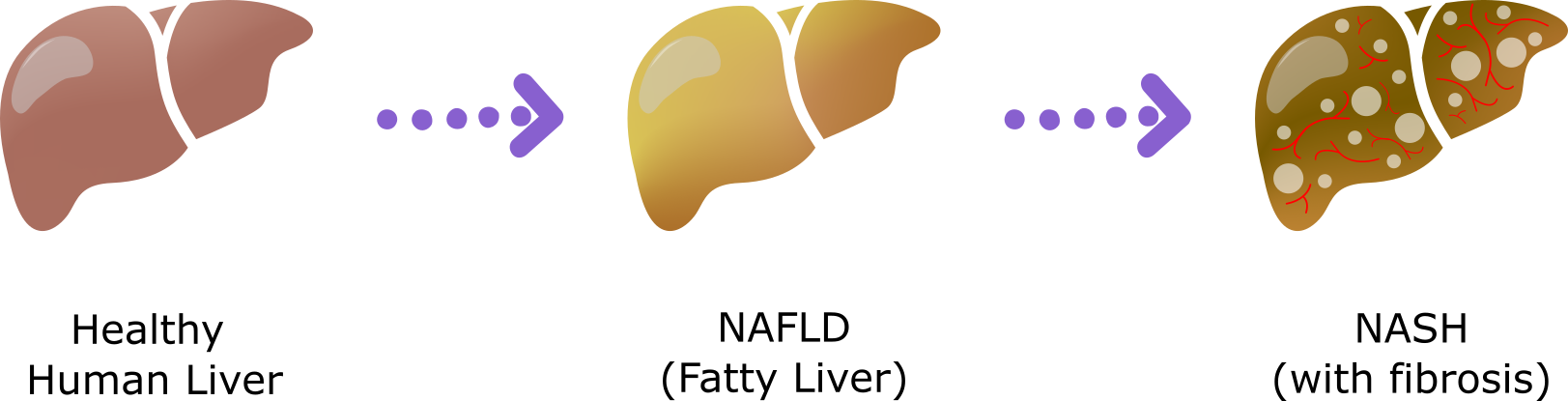
Figure 1: Schematic showing the progression of human liver disease from a healthy human liver to NAFLD to NASH.
The medical community indicates that NAFLD and NASH are emerging public health threats due to the growing prevalence of obesity & type 2 diabetes and faces a number of challenges when looking for treatment. Most patients suffering from NAFLD often show no symptoms until after their livers are already heavily damaged. In addition, there are currently no approved therapeutics for NAFLD, so treatment is centered around weight loss and lifestyle changes. Pharma and biotech companies are investing heavily in NAFLD/NASH research to develop early diagnostic tools and therapies to treat patients who have already progressed to liver fibrosis.
A key challenge in the development of effective NASH therapeutics is the lack of an animal model that resolves the issue of species differences between humans and rodents and accurately recapitulates all features and progression of the human disease[3].
PhoenixBio's Humanized Liver NASH Model
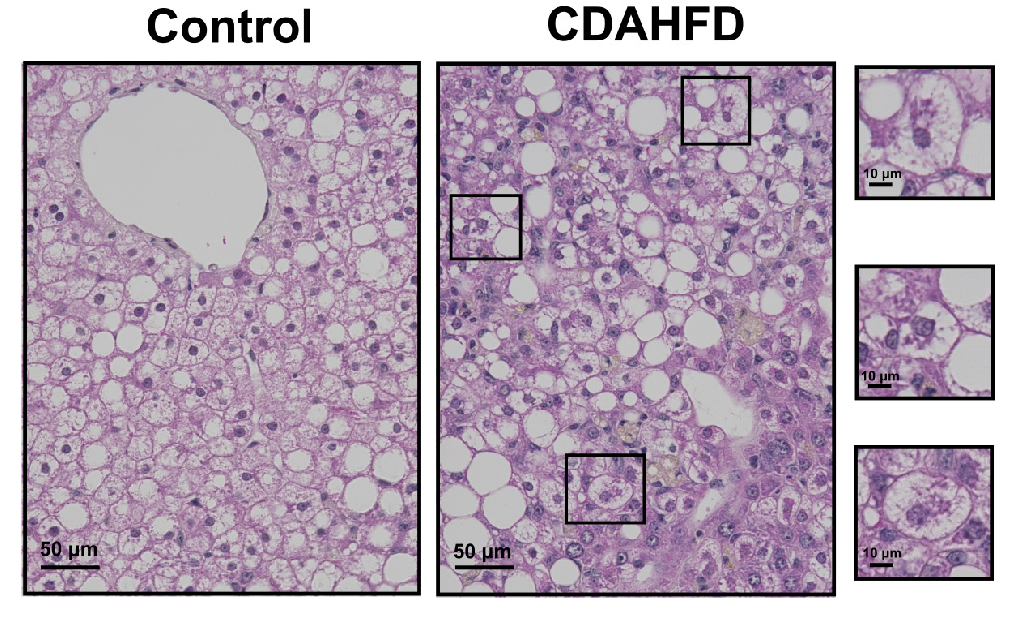
Figure 2: H&E staining reveals Hepatocyte Ballooning and Mallory-Denk bodies (inset boxes) in the human hepatocytes of the PXB-mice fed with the CDAHFD. Adapted from Kisoh et al., 2021.
PhoenixBio’s PXB-mouse® model has up to 95% human hepatocyte engraftment and has been previously shown to have human gene and protein expression, including human-like drug metabolism, lipoprotein profile, and nuclear receptors. Thus, the PXB-mouse® could present an ideal tool for investigating the mechanisms of NAFLD/NASH and developing new drugs.
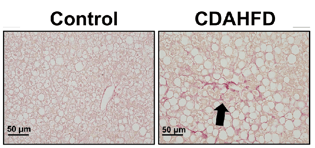
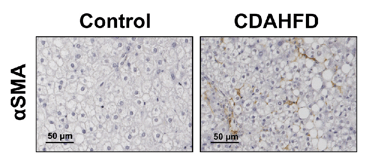
Figure 3: PXB-mice fed with the CDAHFD exhibit fibrosis as confirmed via sirius red positive staining (left), and activation of hepatic stellate cells as confirmed by alpha smooth muscle actin staining (right). Adapted from Kisoh et al., 2021.
The PXB-mouse fed with a high fat diet recapitulates the clinical features of human liver disease, including fibrosis, and is available for applications in NASH/NAFLD.
A new study published in Biomedicines, Kisoh et al.[4] shows that NASH can be induced in PXB-mice with a choline-deficient, L-amino-acid-defined, high-fat-diet (CDAHFD). Animals fed with the CDAHFD showed similar histological changes in the liver as are observed in human NASH patients, including hepatocyte ballooning, inflammation, apoptosis, regeneration of human hepatocytes, and pericellular and perisinusoidal fibrosis. In addition, human hepatocyte injury was evident based on the elevation of plasma human alanine aminotransferase 1 (hALT-1) in these mice.
Efficacy Evaluation
In recent years, a number of NASH therapeutics have failed to meet endpoints in clinical trials, despite good efficacy in pre-clinical mouse models. To determine if the PXB-mouse® NASH model could accurately predict clinical outcomes, Kisoh and colleagues studied the effects of Elafibranor in PXB-mice fed with the CDAHFD. Prophylactic administration of Elafibranor, in conjunction with CDAHFD feeding, improved steatosis and ballooning of hepatocytes, and prevented fibrosis progression. However, in a therapeutic study, treatment with Elafibranor in mice with established NASH pathology following 8 weeks of CDAHFD, did not inhibit liver damage, which was consistent with the results of clinical trials[5].
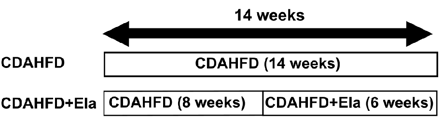
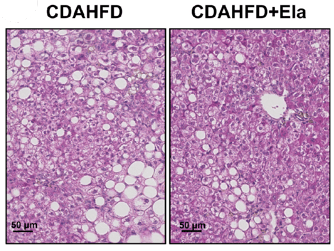
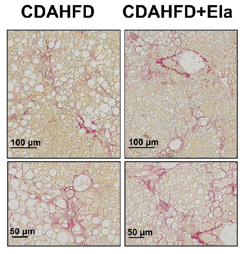
Figure 4: In a therapeutic treatment experiment (top), Elafibranor prevented the progression of steatosis and ballooning (bottom left), but not fibrosis (bottom right). Adapted from Kisoh et al., 2021
The novel CDAHFD-induced NASH model developed by Kisoh et al. using PXB-mice recapitulates key clinical features commonly observed in the early stages of human NASH, including hepatocyte ballooning, inflammation, apoptosis, regeneration of human hepatocytes, and fibrosis. Due to its humanized liver, this model could provide insights into the pathophysiology of human NASH, identify superior biomarkers of disease and better predict therapeutic efficacy in patients.
It should be noted, however, that since the PXB-mouse® has a SCID genetic background, there may be limitations in its application for evaluating therapeutics with an anti-inflammatory mode of action. Nonetheless, the PXB-mouse® NASH model provides an exciting new preclinical option to further our understanding of the mechanisms driving human NASH development and accelerate the development of novel treatments for this serious and increasingly prevalent disease.
References
-
Younossi et al., 2016. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64(1):73-84. doi: 10.1002/hep.28431. Epub 2016 Feb 22. PMID: 26707365.
-
Younossi et al., 2019. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation. 2019 Jan;103(1):22-27. doi: 10.1097/TP.0000000000002484. PMID: 30335697.
- Santhekadur er al., 2018. Preclinical models of non-alcoholic fatty liver disease. J Hepatol. 2018 Feb;68(2):230-237. doi: 10.1016/j.jhep.2017.10.031. Epub 2017 Nov 9. PMID: 29128391; PMCID: PMC5775040.
-
Kisoh et al., 2021. Estimating Drug Efficacy with a Diet-Induced NASH Model in Chimeric Mice with Humanized Livers. Biomedicines. 2021 Nov 9;9(11):1647. doi: 10.3390/biomedicines9111647. PMID: 34829876; PMCID: PMC8615377
-
https://ir.genfit.com/news-
releases/news-release-details/ (Accessed Dec 2021)genfit-announces-results- interim-analysis-resolve-it- phase-3
